Activity Contour Diagram
Purpose: Learn to calculate activity contour diagram. In this example, the activity contour lines of Mg will be calculated and plotted on the isothermal section of the Al-Mg-Zn ternary system at 500 °C.
Module: PanPhaseDiagram
Thermodynamic Database: AlMgZn.tdb
Batch file: Example_#1.15.pbfx
Calculation Procedures:
-
Load AlMgZn.tdb following the procedure in Pandat User's Guide: Load Database , and select all three components;
-
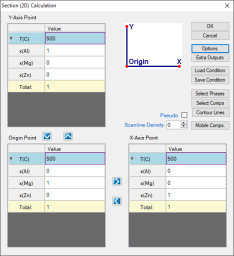
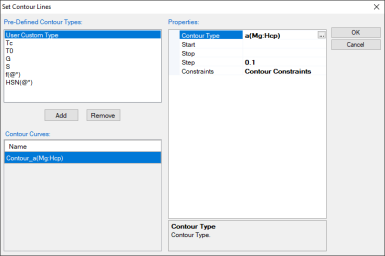
First set up the calculation condition as shown in Figure 1 in the same way as we did to calculate Al-Mg-Zn isotherm (see Isotherm of Al-Mg-Zn at 500°C), then click “Contour Lines” to open the contour line dialog as shown in Figure 2;
-
Choose “User Custom Type” and click AddType in “a(Mg:Hcp)” for the Contour Type in the “Properties” window as shown in Figure 2, then press OK;
Figure 1: Setup calculation for an isotherm in the Al-Mg-Zn ternary at 500°C, then press “Contour Lines”
Post Calculation Operation:
-
Change graph appearance following the procedure in Pandat User's Guide: Property;
-
Label each line by putting the cursor on each line and wait for the tool tip to pop out, then press F2;
Information obtained from this calculation:
-
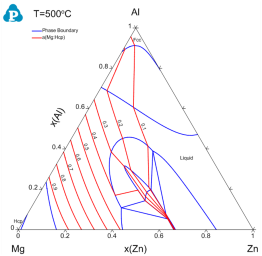
Figure 3 shows the activity contour diagram of Mg at 500 °C. The variation of Mg activity in the composition triangle is clearly seen at this temperature;
-
Activity contour lines can also be plotted only in the selected phase field, such as in the Liquid phase field, in the Liquid+Fcc phase field. An example is given in Pandat User's Guide: Activity Contour Diagram ;